A Chemical System At Equilibrium Is Referred To As Dynamic Because The
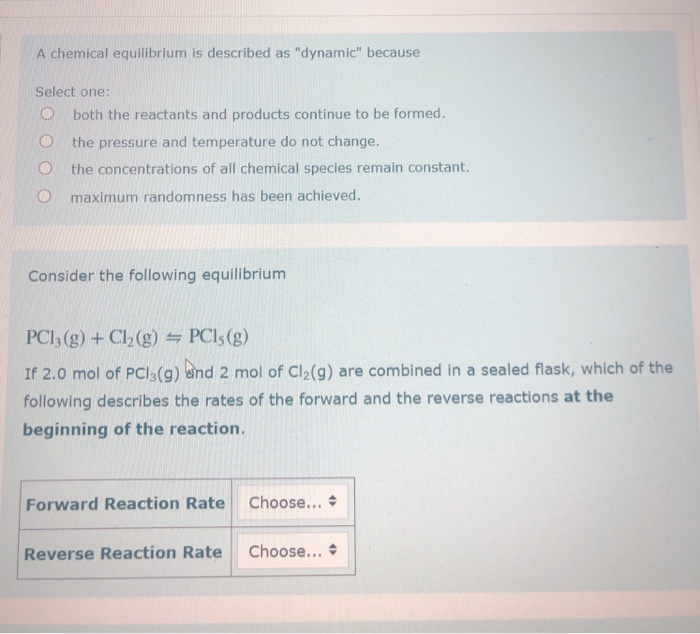
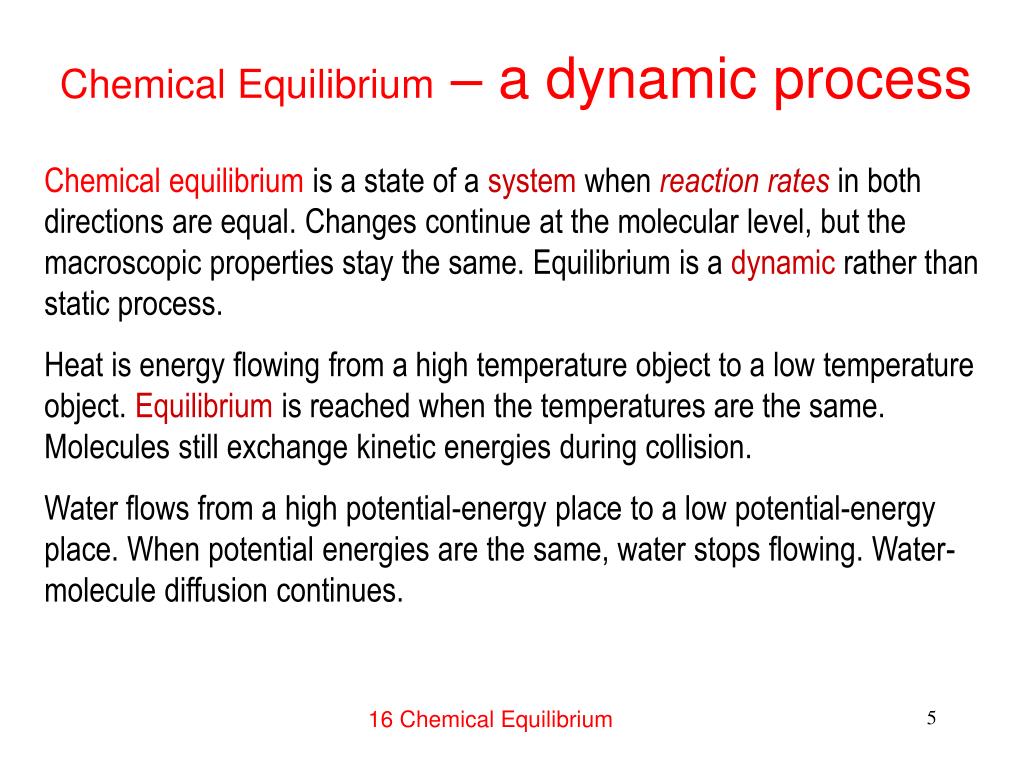
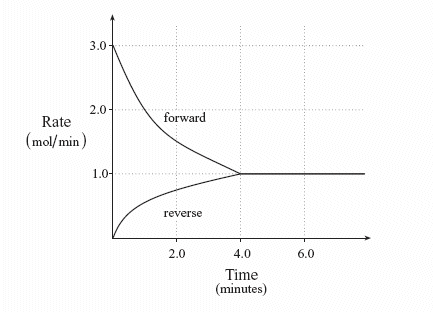
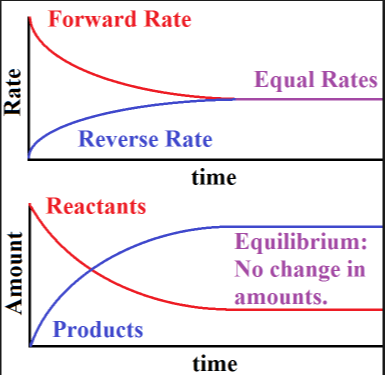
A chemical system at equilibrium is referred to as dynamic because the. The rates of the forward and reverse reactions continue changing. A system is said to be in dynamic equilibrium when there is no longer any net change in the concentrations of products or reactants. A chemical reaction in which the rate of the reactants is equal to the rate of backward products.
Group of answer choices. Chemical equilibrium is dynamic process because in it rate of forward reaction is equal to rate of backward reaction. It is dynamic because there are many factors that affect what that ratio will be as defined by LeChatelier.
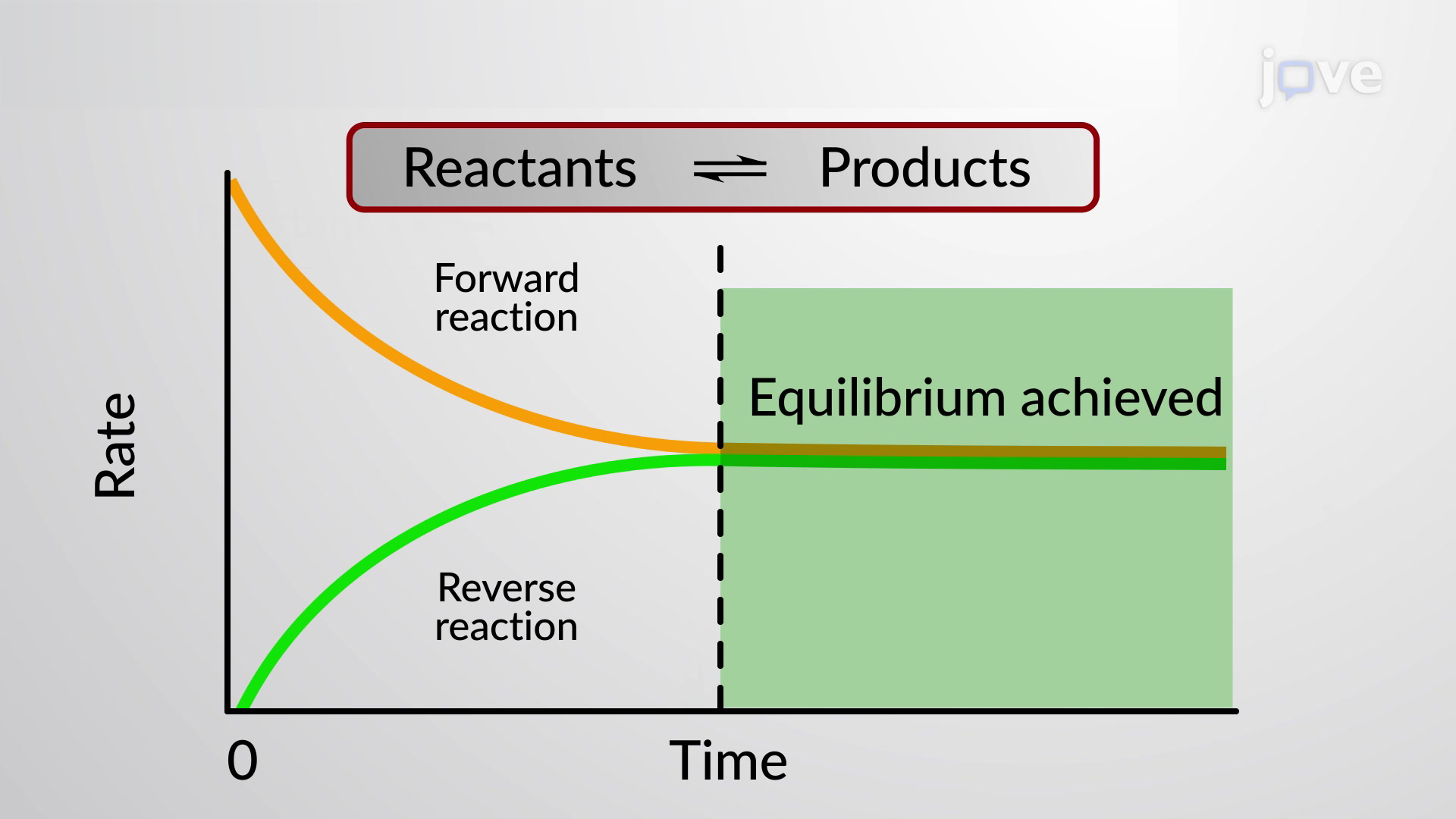
These equations are dynamic because the forward and reverse reactions are still occurring but the two rates are equal and unchanging so theyre also at equilibrium. 1 on a question Achemical system at equilibrium is referred to as dynamic because the a rates of the forward and reverse reactions continue changing b forward and reverse reactions continue reac - the answers to brainsanswerscouk. The reaction hasnt stopped but both forward and backward reactions are still occurring.
The rates of the forward and reverse reactions continue changing. When a chemical system is at equilibrium _____. A reaction is at equilibrium when the amounts of reactants or products no longer change.
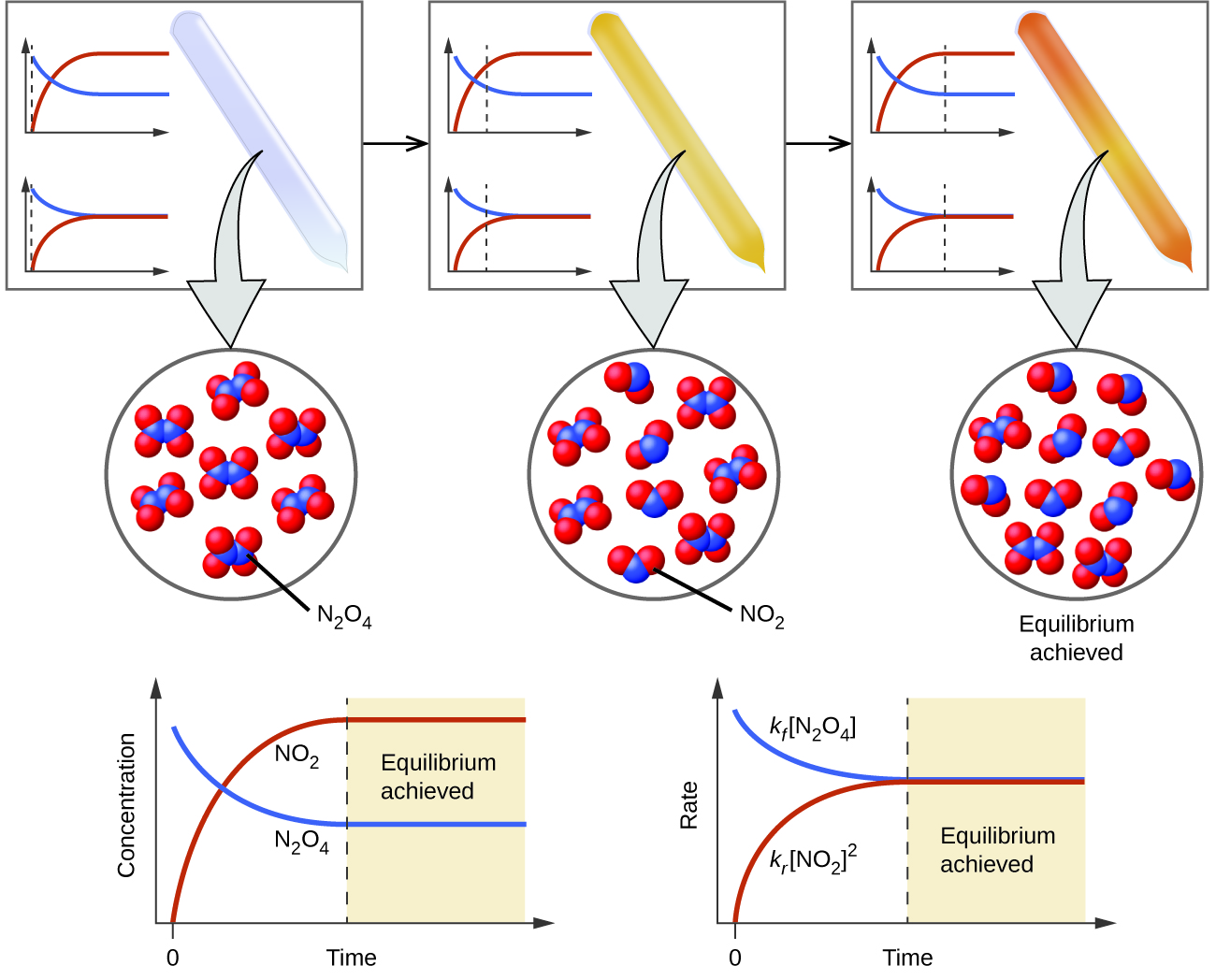
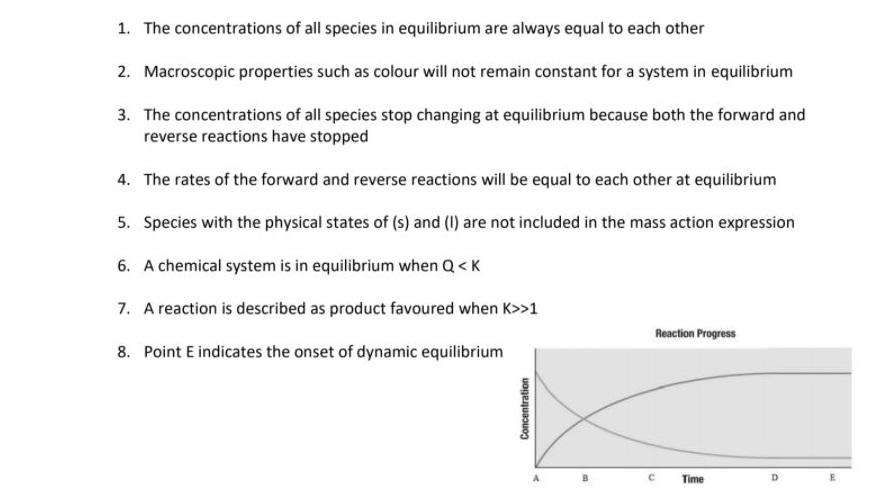
The value of the equilibrium constant K continues changing. In a chemical equilibrium the forward and reverse reactions do not stop rather they continue to occur at the same rate leading to constant concentrations of the reactants and the products. A chemical system at equilibrium is referred to as dynamic because the A rates of the forward and reverse reactions continue changing B forward and reverse reactions continue reacting C value of the equilibrium constant K changes D concentrations of the components continue changing.
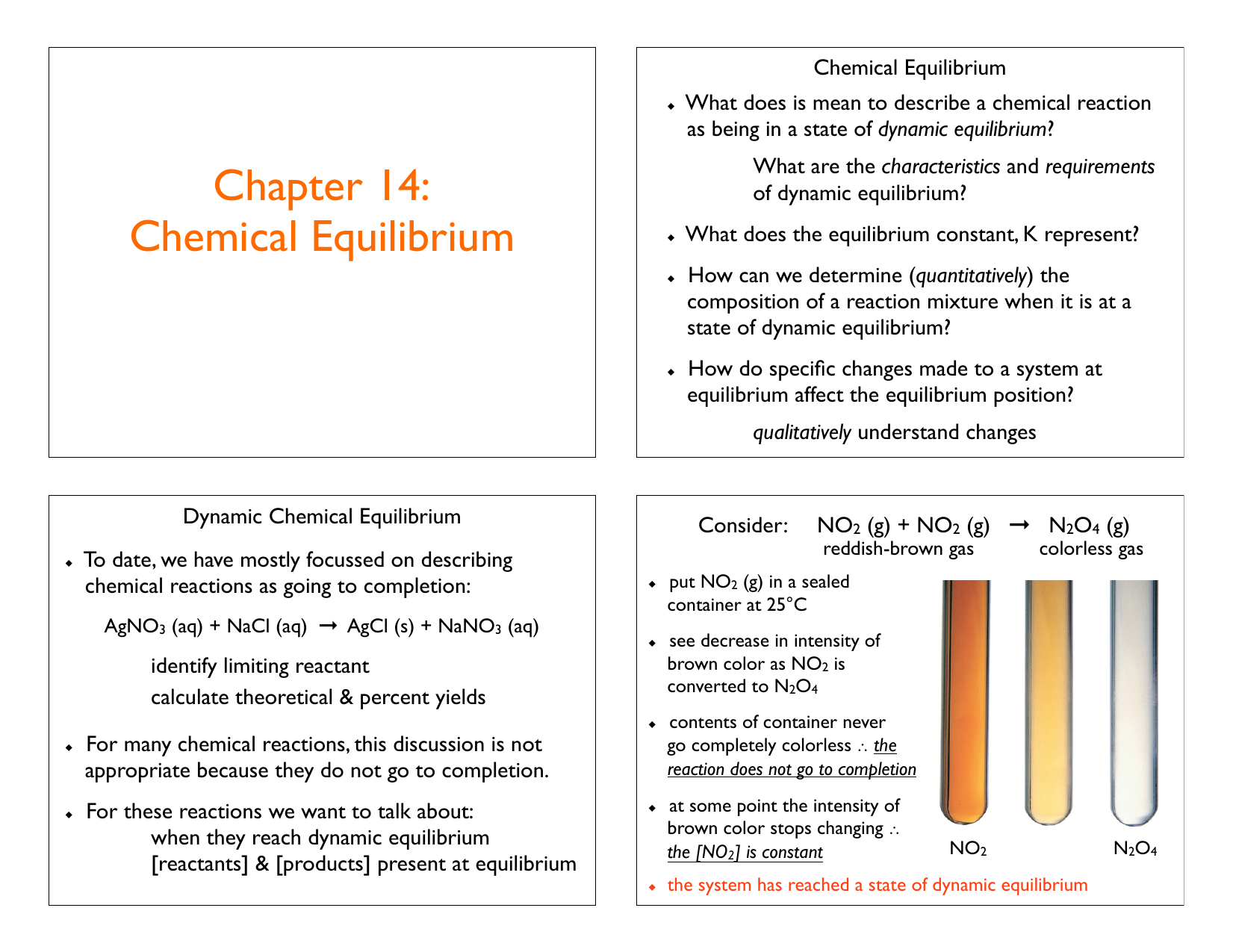
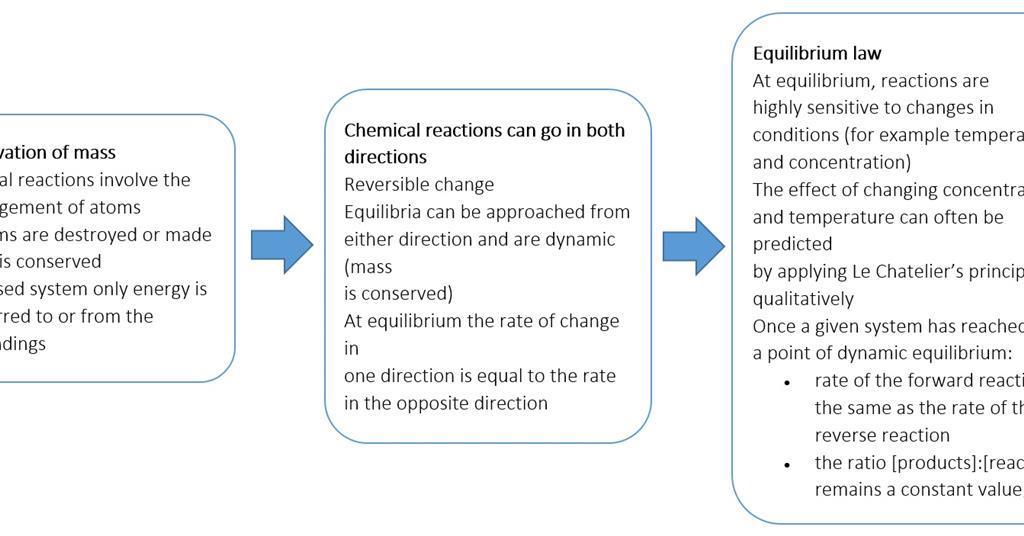
Chemical Equilibrium a Dynamic Equilibrium. In general dynamic equilibrium can occur during a physical change or a chemical change that is reversible. Therefore the dynamic equilibrium can be defined as.
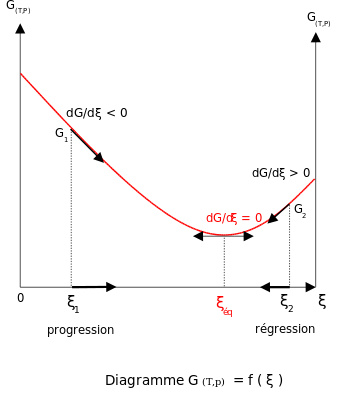
A a reaction in which the free energy at equilibrium is higher than the energy content at any point away from equilibrium B a chemical reaction in which the entropy change in the reaction is just balanced by an opposite entropy change in the cells surroundings. As both reactions are still occurring the equilibrium.
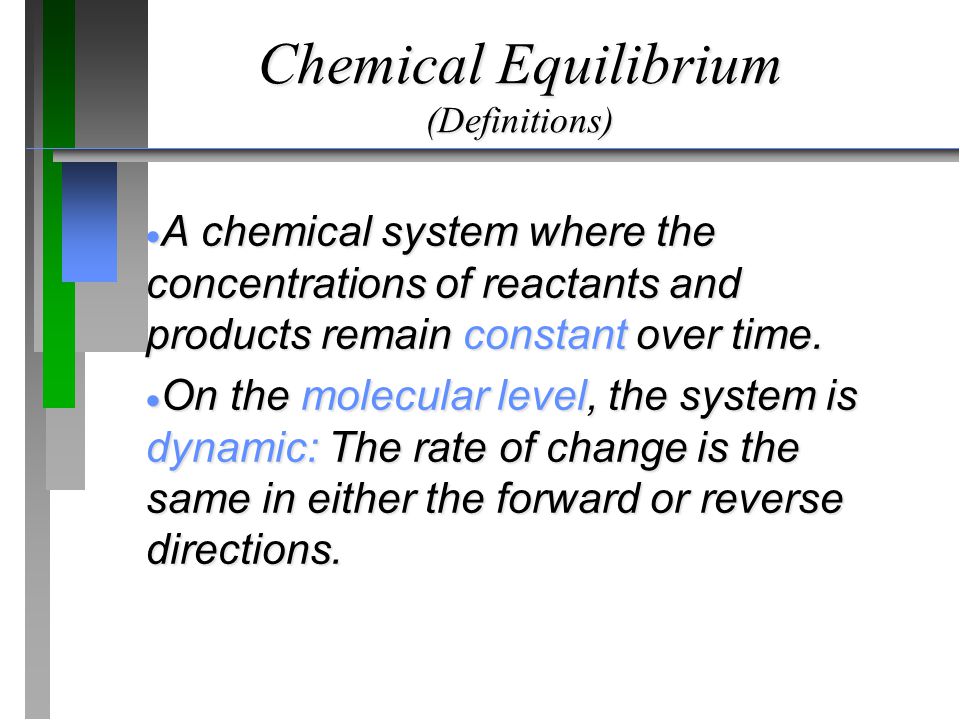
In a chemical equilibrium the forward and reverse reactions do not stop rather they continue to occur at the same rate leading to constant concentrations of the reactants and the products.
In other words A reaction is said to be at dynamic equilibrium when the reactants are converted into products and the products are converted to reactants at an equal and constant rate. It is a chemical reaction where the reactants form products that in turn react together to give the reactants back. As both reactions are still occurring the equilibrium. Why is a chemical system at equilibrium referred to as dynamic. A system is said to be in dynamic equilibrium when there is no longer any net change in the concentrations of products or reactants. The value of the equilibrium constant k continues changing. Dynamic equilibrium involving a physical change is referred to as physical equilibrium while that involving a chemical change is known as chemical equilibrium. The rates of the forward and reverse reactions continue changing. And in chemical equilibrium the reaction is going on but the rate of formation of reaction is equals to the rate of formation of product.
A reaction is at equilibrium when the amounts of reactants or products no longer change. When a chemical system is at equilibrium _____. Chemical equilibrium is a dynamic process. Why is a chemical system at equilibrium referred to as dynamic. A a reaction in which the free energy at equilibrium is higher than the energy content at any point away from equilibrium B a chemical reaction in which the entropy change in the reaction is just balanced by an opposite entropy change in the cells surroundings. The reaction hasnt stopped but both forward and backward reactions are still occurring. And in chemical equilibrium the reaction is going on but the rate of formation of reaction is equals to the rate of formation of product.








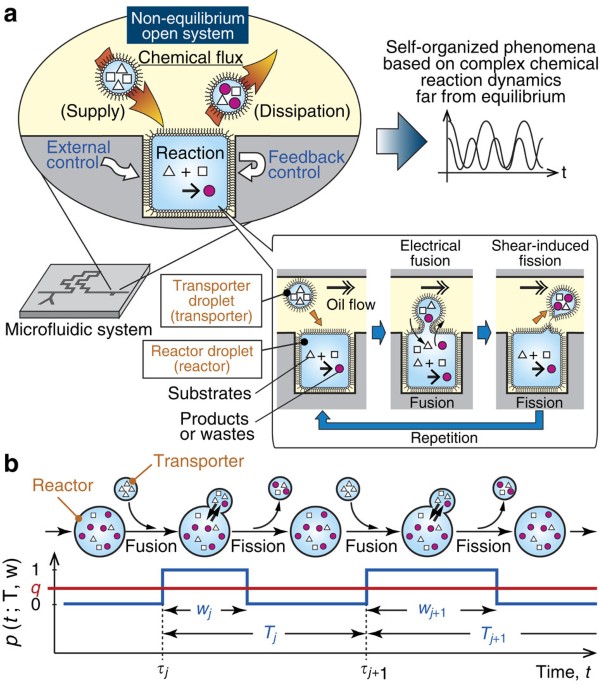
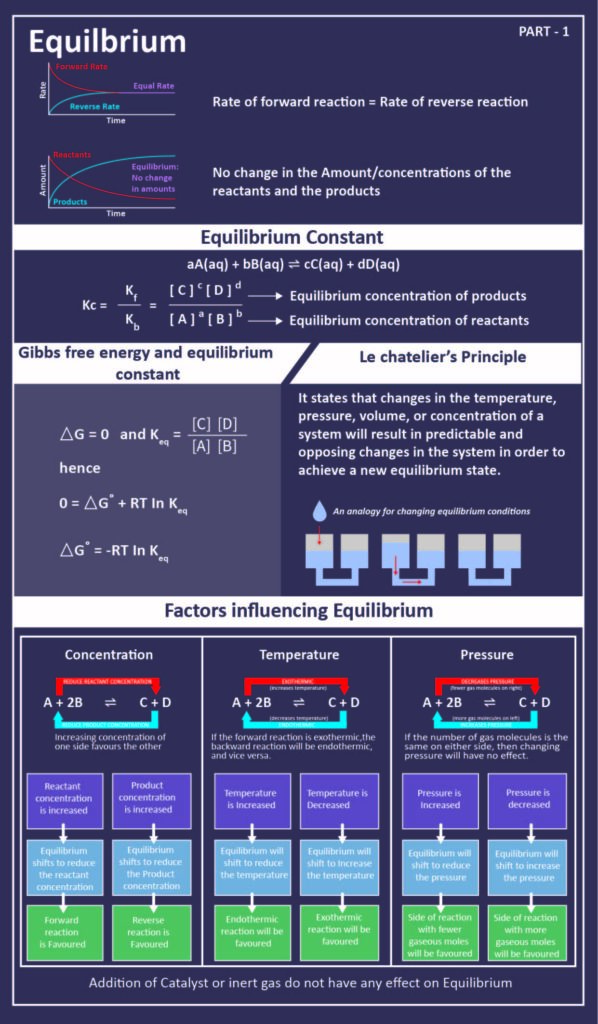





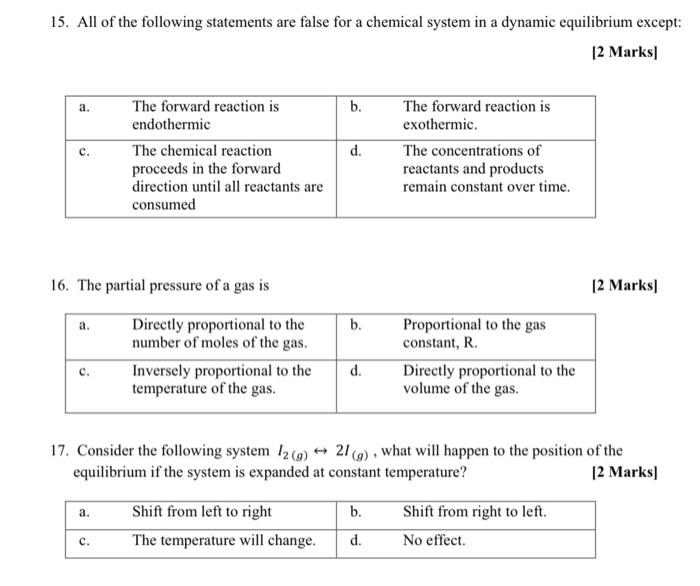


















Post a Comment for "A Chemical System At Equilibrium Is Referred To As Dynamic Because The"